Breaking News


Fast, Reliable, and Uncensored News Coverage
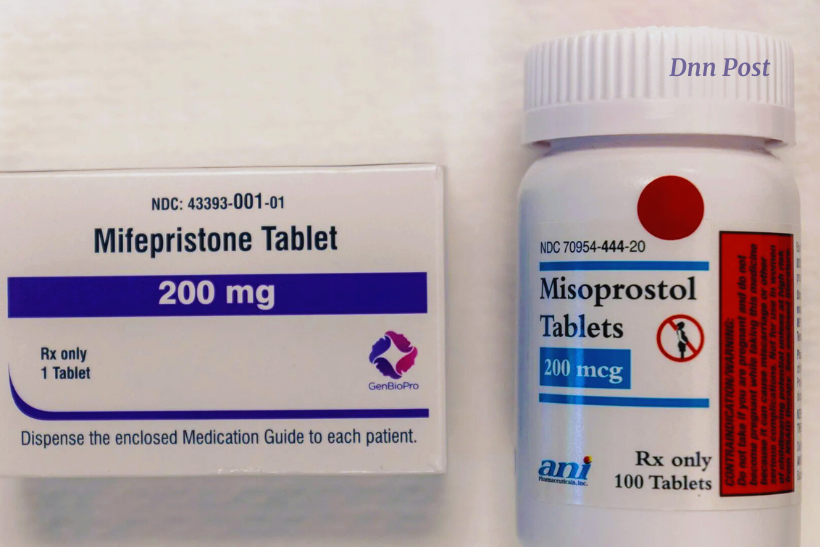
The U.S. Food and Drug Administration has approved a second generic version of mifepristone, reigniting controversy as conservative groups immediately denounced the decision, according to reports from The Hill
The newly approved generic, produced by Evita Solutions, is deemed “bioequivalent” to the brand-name drug and slated for market release in early 2026, per regulatory filings.
The FDA stated that its role is limited to evaluating formula equivalence, not endorsing the product. Critics counter that the timing is politically charged, especially given prior pledges by Health officials to review mifepristone’s safety.
Anti-abortion leaders swiftly condemned the approval. Senator Josh Hawley said he had “lost confidence in leadership at FDA,” while advocacy groups called the decision a betrayal of conservative principles. They argue that approving the drug before completing promised safety reviews undermines transparency and trust.
We expected thorough scrutiny before expanding access,” one anti-abortion advocate said, pressing that due diligence must supersede speed.
The approval intensifies a fraught debate over reproductive rights heading into an election year. Watch for legal challenges, congressional probes, and efforts from states to either restrict or defend access to the drug.